Reaction can take place quickly or slowly depending on the nature of the substances involved. If you tried to form rust from some iron, it can take an average of two weeks. On the other hand, if you put hydrochloric acid in the presence of sodium hydroxide you will get a reaction that produces salt and water in seconds. These worksheets will look at all the different types of chemical reaction you will see in any science curriculum. This group of sheets really focuses on understanding how a reaction can be understood from the molecular masses all the way through to diagramming the reaction that takes place.
Print Chemical Reactions Worksheets
Click the buttons to print each worksheet and associated answer key.


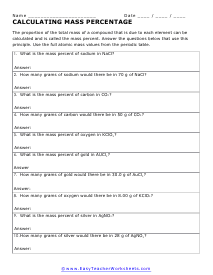
Percentage Mass
The proportion of the total mass of a compound that is due to each element can be calculated and is called the mass percent.



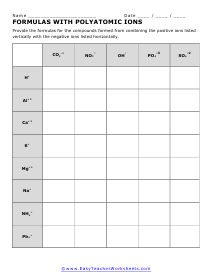
Determining Formulas of Polyatomic Ions
What compound forms? For extra credit name the compound in words.

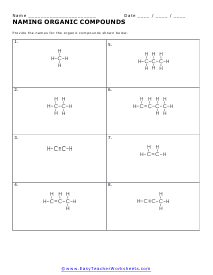
Name Non-Binary Compounds
These are a little more difficult. Make sure to read everything throughly.





Reaction Types
Classify each chemical reaction as either a decomposition, single replacement, double replacement, or synthesis.

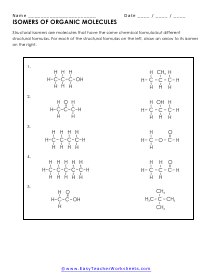
Isomers of Organic Molecules
Structural isomers are molecules that have the same chemical formula, but different structural formulas.


What Are Reactions?
Reactions rates can be sped up with the application of energy like heat, sunlight, or electricity or by increasing the concentration or pressure of the reactants.

What Are They? Questions
Alternatively, the addition of a third substance called an inhibitor can close down a reaction.


QUESTIONS: Synthesis Reactions
Are the reactants in a synthesis reaction typically individual elements or compounds?

Decomposition Reactions
In a decomposition reaction one more complex substance breaks down to form two separate, simpler substances.

QUESTIONS: Decomposition Reactions
Most decomposition reactions are endothermic. In an endothermic reaction, energy is applied in the form of heat, electricity, or sunlight in order to break down the bonds of a more complex molecule.

Combustion
Combustion was the first chemical change discovered by man. The ability to use and control fire helped early humans to survive and revolutionized the way that they lived.


Single and Double Displacement Reactions
A single displacement reaction is also known as a single replacement reaction or a substitute reaction.

QUESTIONS: Single and Double Displacement Reactions
In a double displacement reaction, also known as a metathesis reaction, the compounds on the left side of the equation switch substances.

Photochemical Reactions
A photochemical reaction is a chemical reaction in which the heat needed to induce a chemical reaction comes from molecules of light called photons.

QUESTIONS: Photochemical Reactions
What is the name of the structures plants use to capture sunlight?

Catalysts and Inhibitors
A common way to add a catalyst to a chemical reaction is to add energy in the form of heat, sunlight, or electricity.

QUESTIONS: Catalysts and Inhibitors
An inhibitor is the opposite of a catalyst. Adding an inhibitor increases the amount of activation energy that is needed in order for a chemical reaction to occur.

Reactants, Reagents, and Products
A reactant is a substance that is present at the beginning of a chemical reaction and that is changed by the reaction to create a new substance called the product.

QUESTIONS: Reactants, Reagents, and Products
How and where do you note if a reaction has taken place under certain circumstances?

Acid-Base Reactions
In chemistry, an acid is a substance that can release a proton, and a base is a substance that can receive a proton.

QUESTIONS: Acid-Base Reactions
The most often performed school science experiment is an acid-base reaction: the volcano experiment in which you add vinegar to baking soda.

Oxidation-Reduction Reactions
A redox reaction always take place in two parts: a reduced half and an oxidized half. These two parts always occur together.

QUESTIONS: Oxidation-Reduction Reactions
What kind of reactions are typically (but not always) redox reactions with oxygen being oxidized?
What Causes Chemical Reactions?
Every day, different chemical reactions happen around us – cooking eggs, lighting a fire, and more. Each of them is a unique set of circumstances, but they have a general cause.
Chemical reactions are caused by the breaking of bonds in reactants to form new bonds of products. Energy is needed to form bonds but is released when bonds are broken. In a reaction, the amount of matter is preserved regardless of changes in structure.
Keep reading to learn more about chemical reactions, such as different types and physical indicators.
What Is a Chemical Reaction?
Chemical reactions vary a lot. Some occur quickly, while others need a catalyst. Some have apparent changes, while others don’t. But they are all processes that lead to products.
A chemical reaction is the conversion of substances, called reactants, into new compounds, called products. These products have new structures that are caused by breaking and forming bonds. There are different types of chemical reactions.
Reactions can either be reversible or not. In reversible forms, products can reform into the original reactants, given the right conditions. Thus, it can move in two directions: forward into products or backward into reactants.
Contrary to reversible forms, irreversible ones can only move forward. Thus, products cannot form back into reactants.
Chemical reactions are happening all the time around use and we just don’t realize it. Ever clean your kitchen with something other than water? Ever rinse with mouth wash? Have you seen the glorious baking soda and vinegar volcano that is so popular at science fairs? They happen when molecules from one substance break apart and combine, to some degree, with another. This usually is irreversible and forms a new substance all together. Some of them give off heat, like hand warmers. Some take in heat (get colder). Instant ice packs are an example of that. Others give off brilliant colors. Where do you think firework reactions come from?
What Are Physical Indicators of a Chemical Reaction?
While not applicable to all reactions, many processes display physical indicators of a chemical reaction.
These are the most common indicators:
- Change in color
- Change in temperature
- Production of light
- Production of gas
- Formation of precipitate
These indicators are the result of changes in chemical structure.
For instance, exothermic reactions, which involve breaking bonds, are accompanied by signs of increased temperature because energy was released.
On the other hand, endothermic types have reduced temperatures because they absorb energy instead of releasing them.
What Are the Basic Types of Chemical Reactions?
Thousands of them occur every day. While they may be all unique in one way or another, they can still be categorized into general types.
The following are general descriptions of the transformation that takes place. Every reaction can be categorized into a basic type.
These are the basic types these of chemical reactions:
- Combination/SynthesisTwo or more reactants combine to form one new product. General Equation: A + B → AB
- DecompositionA reactant separates into two or more substances. It requires energy. General Equation: AB → A + B
- Single-ReplacementOne element reactant replaces a similar element in a compound. General Equation: A + BC → AB + C
- Double-ReplacementIons in a compound exchange places to form new ones. General Equation: AB + CD → AD + CB
- CombustionCompounds react with oxygen and produce gas and light. General Equation: CwHx + O2 (g) → yH2O (l) + zCO2 (g)
What Are Other Types of Chemical Reactions?
Other forms, such as acid-base reactions, are more specific regarding the components, mechanisms, and products.
These are a few examples of other types of chemical reactions:
- Acid-baseThese are common and essential reactions. It involves the exchange of ions or electrons to produce new compounds. It may also be a form of neutralization reactions (i.e., bases neutralize acids to form salt and water).
- PrecipitationDissolved reactants form a solid product in precipitation reactions. A precipitation reaction is a kind of double-replacement reaction. Precipitates will develop depending on the solubility properties of reactants.
- RedoxRedox reactions, or oxidation-reduction reactions, involve the transfer of electrons that lead to ionic products. A combustion reaction is an example of a redox reaction.
- HydrolysisHydrolysis reactions use water as one of the reactants. Smaller products are formed from these reactions.
- Condensation Contrary to hydrolysis, water is a product of condensation. Two substances combine to create a larger compound with water as a byproduct.
Final Thoughts
Chemical reactions occur because of changes in bond structure – either new ones are formed, old ones are broken, or both. In the process, energy may be released or used.
There are signs of such reactions, like color or temperature changes, but not all of them have apparent indicators. Each reaction can be categorized into basic or more specific types.


