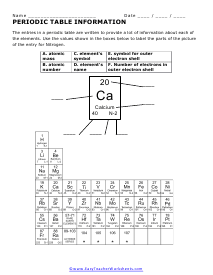
Noted scientist Dmitri Mendeleev was touted as creating the first version of the Periodic Table. He showed that a certain arrangement of elements could lead us to understand the properties of other elements that were related to each other. He also used it to predict the properties of elements that were either not discovered yet or not normally found in nature. Elements on the table are ordered by their number of protons which is equivalent to their atomic number. The elements on the table can be found at room temperature in all of the various phases. Students can use the Periodic Table to help them predict the outcome of chemical reactions and the stability of compounds in the modern science classroom. Elements that exist in the same column of the table tend to not only similar chemical properties, but also form similarly charged ions. As you progress from right to left in a row of elements several trends emerge: the amount of energy required to remove an electron increases, the radius of an atom of the element decreases, as well as the increased tendency to attract a pair electrons. This really helps us also write the chemical formulas for compounds and balance chemical equations.
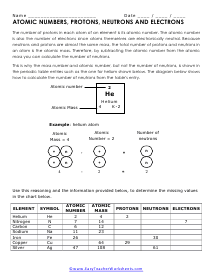
The worksheets range from finding basic information about particular elements that are found in nature to working on determine atomic operations such as neutron numbers. These worksheets start by thoroughly explaining the information that can be found on a single atom of an element on the table. We then look into all the compositional math that exists for these elements. We show you how to determine the number of protons, neutrons, and electrons found in a stable atom of each element. As we progress, we will present students with fun puzzles that helps sum up everything we can learn from this marvel of science.